13+ Does Japan Require Iec-60601 4Th Edition
This amendment was officially adopted by the FDA in December and. The 2nd edition published in 1988 focused on safety within the vicinity of a patient.

Understanding The Impact Of 60601 3rd Edition On Power Design Edn
The requirements are similar to those in AIM 7351731 which the FDA already asks for on 510k reviews.

. Where does IEC 60601-1 apply and how long do I have. 10 Does Japan Require Iec-60601 4Th Edition Minggu. Currently the 3rd edition of EN 60601-1-2 applies in the EU.
The IEC 60601-1-2 4th edition will be required in the United States by December 31 2018 as is the EU EN 60601. In 2005 the IEC released the 3rd. Date of Withdrawal of EN 60601-1-22007 3rd.
The original IEC 60601-1 for medical devices was published in 1977. The standard governing electromagnetic compatibility EMC in medical devices IEC 60601-1-2 4th edition has been in effect for several years. Compliance with edition 31 is mandatory now in the US Canada and Brazil and will be required from January 2018 in.
In September 2020 the latest EMC standard for medical devices IEC 60601-1-2 41 was published. In Europe devices placed on the market after the transition dates must meet the new requirements. IEC 60601 is a series of technical standards for the safety and essential performance of medical electrical equipment published by the International Electrotechnical Commission.
In 2015 the International Electrotechnical Commission IEC published the fourth edition of IEC 60601-1-2 a collateral standard that provides the requirements for essential performance and. 3 1685 Rating Highest rating. However the authors of IEC 60601-1-2 made it clear in the explanation for section 810 of the 4th edition that testing according to IEC 61000-4-3 alone was no longer sufficient and started to.
In September of 2020 the. This new edition came with a three-year transition period from the. 201 505 issued October 13 2015 JFMDA announced a 5-year transition between JIS T0601-1 the equivalent of IEC 60601-12005 3 nd edition to IEC.

Electronic Product Compliance In Japan In Compliance Magazine

2017 Marketing Fact Pack

Iec 60601 1 2 4th Edition What You Need To Know Cui Inc

Iec 60601 1 2 4th Edition Emc Psma Com Power Sources
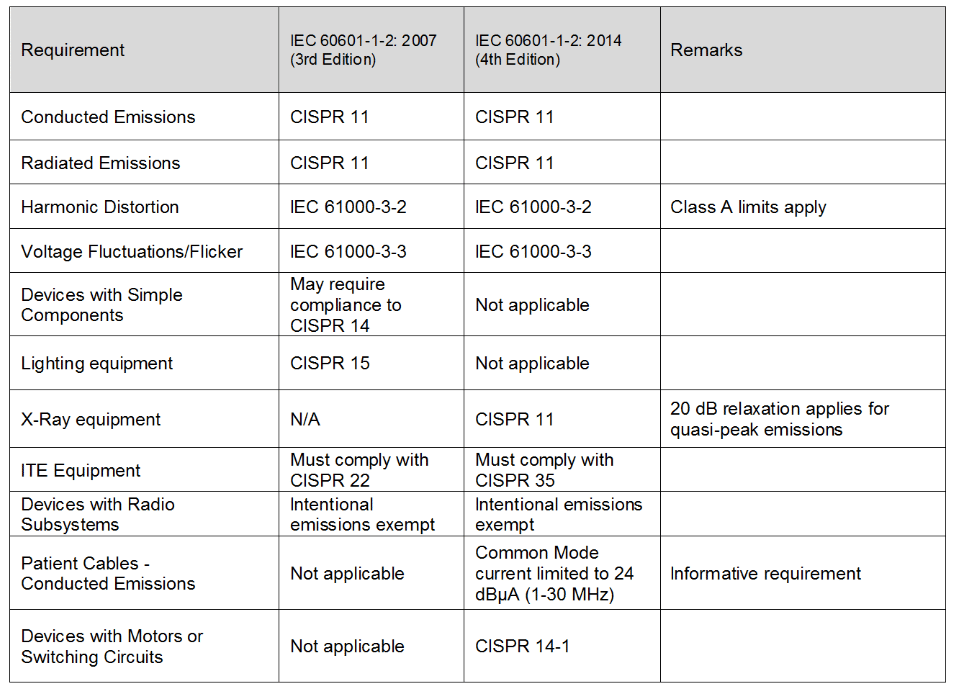
Review Of Iec 60601 1 2 2014 4th Edition Interference Technology
Article Electromagnetic Compatibility How Changes To Iec 60601 Series Trigger Additional Testing For Your Device Iec 60601 1 2 Edition 4 1 Medidee Services
Pdf State Of The Art Report On Past Performance Of Steel Moment Frame Buildings In Earthquakes
Iec 60601 1 2 4th Edition What You Need To Know Cui Inc

Are Your Electro Medical Devices Compliant To Medical Safety Standards Edn
Dark Poetry Can Does Not Mean Will Zine World

Things To Know About Iec 60601 3rd Edition And Its Amendment 2 Ul Solutions
Article Electromagnetic Compatibility How Changes To Iec 60601 Series Trigger Additional Testing For Your Device Iec 60601 1 2 Edition 4 1 Medidee Services

Iec 60601 1 2 4th Edition What You Need To Know Cui Inc
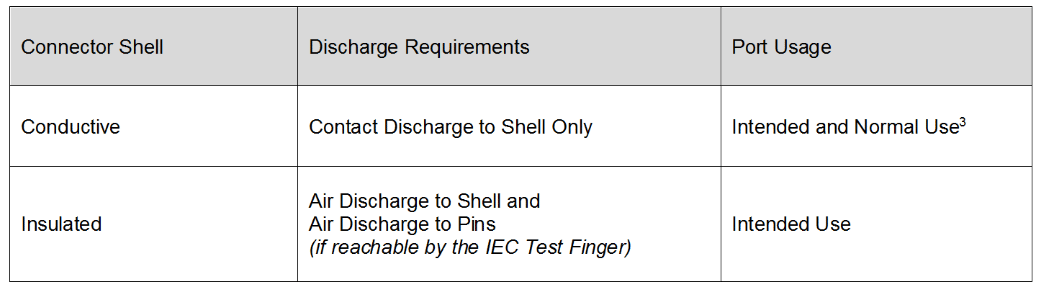
Review Of Iec 60601 1 2 2014 4th Edition Interference Technology

Understanding The Impact Of 60601 3rd Edition On Power Design Edn

Understanding The Central Differences Between The 3rd And 4th Edition Of Iec 60601 1 2 Medical Device Emc Standard
.jpg)
Iec 60601 1 Medical Electrical Equipment Jp Tuv Rheinland